APPLICATIONS TO ACHIEVE MORE
High-Throughput Screens
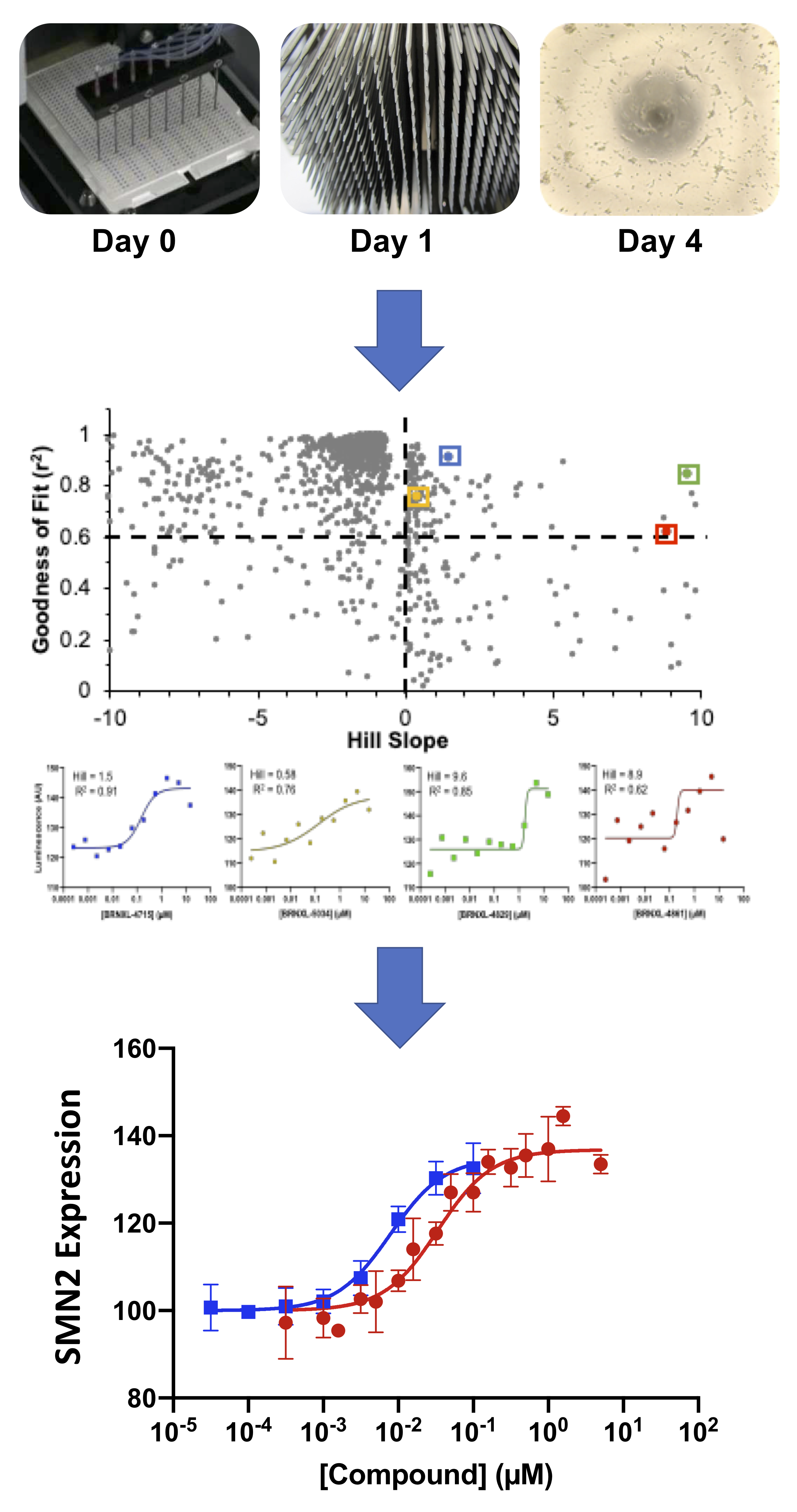
The lack of therapeutics for devastating CNS diseases and the high failure rate of promising new drugs in clinical trials argue for the need to improve drug discovery strategies. Improving clinical relevance along the drug discovery pipeline can increase the likelihood of clinical success. Using BrainXell’s patient iPSC-derived neurons in high-throughput screens for new therapeutics improves the clinical relevance at the initial stages of drug discovery. For example, spinal muscular atrophy (SMA) patient motor neurons were used in phenotypic screening for small molecules that can upregulate expression of endogenous SMN2 protein, a promising strategy for treating this fatal disease. More than a billion spinal motor neurons were produced to screen more than 500,000 analytes in 1536-well format. Such a large screen has never been performed using patient-derived neurons, and several technological innovations were developed to enable this high-throughput screen: 1) genetic engineering of patient derived iPSCs to generate a HTS compatible readout of SMN2 expression, 2) large and consistent production of neurons (10^9 motor neurons per batch), and 3) consistent plating of rapidly maturing neurons across hundreds of 1536-well plates. These innovations can be applied to other cortical or motor neuron disease drug discovery in an effort to improve clinical translatability (and success) at the earliest stage of drug discovery
Read more about high-throughput screens:
• Human Motor Neuron Based High-Throughput Screening Platforms for Neurodegenerative Diseases
• SMA Drug Discovery with iPSC-derived Neuron Reporter System
• ALS Drug Discovery with iPSC-derived Neuron Reporter System
Electrophysiology of Neurons
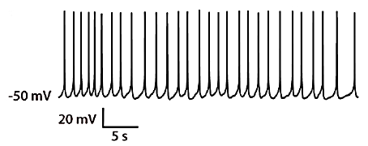
Functioning neurons have precisely controlled membrane potentials and electrical currents flowing through ion channels. These electrical properties are integral to their role in communicating signals throughout the nervous system. Are you interested in studying pathphysiology, pharmacology, or the molecular and cellular processes that govern signaling? Electrophysiology techniques are used to experimentally probe the electrical properties of neurons. A variety of techniques are employed to directly or indirectly measure changes in electrical currents that flow across cell membranes or changes in the electrical potentials near the cell membranes.
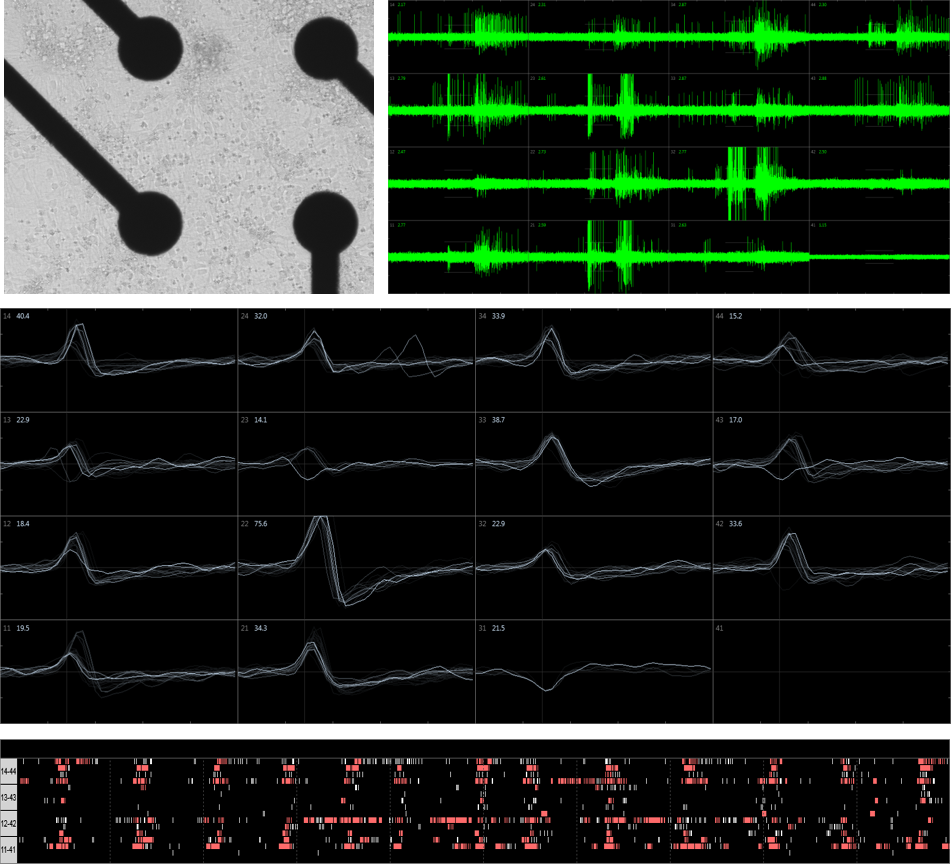
Whole-cell recordings and patch recordings are obtained using specialized recording electrodes and amplifiers that make it possible to measure voltage changes while clamping the current, or measure currents while holding the voltage constant. These techniques allow for the study of ion channels, voltage sensitive proteins, and functional responses to neuro-active compounds. An example is shown of a whole cell recoding in current-clamp mode on BrainXell’s Spinal Motor Neurons (BX-0100) that exhibit spontaneous action potentials.
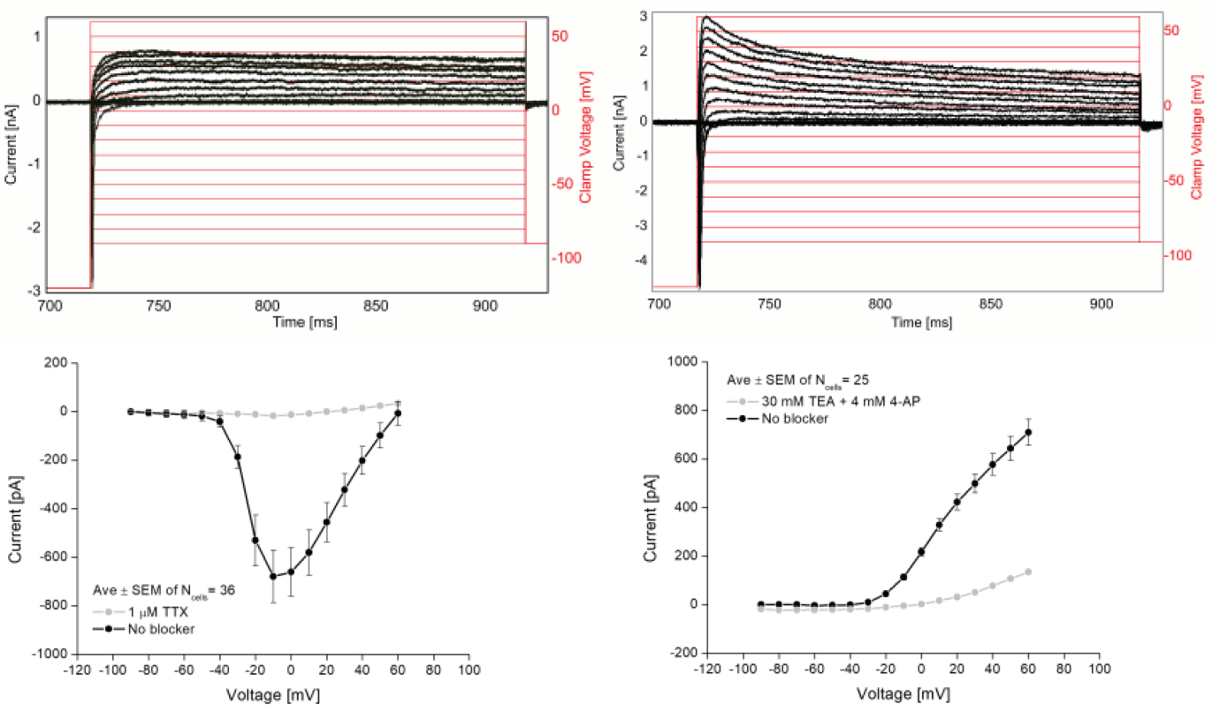
Whole-cell recordings and patch recordings are tedious techniques that require significant time for data acquisition. However, automated platforms are commercially available that offer improved throughput. As demonstrated here using a Qube 384 (Sophion), the electrophysiological properties of hiPSC-derived motor neurons produced by BrainXell can be characterized and Current-Voltage (IV) relationships for both sodium currents and potassium currents can be measured for dozens of cells.
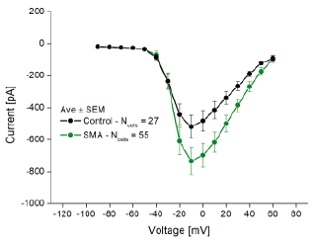
The Qube 384 was also used to compare the phenotypes of SMA disease motor neurons to wild-type motor neurons. The NaV IV relationship of the SMA motor neurons displayed greater peak NaV current as compared to the control.
Extracellular recordings, which examine changes in electrical field potentials created by neurons that are adjacent to recording electrodes, can also be used to observe neuronal activity. This technique often utilizes Multi-Electrode Arrays (MEA) that record extracellular action potentials (spikes) from numerous neurons at the same time, allowing networks and synchronized signaling to be studied. Such recordings are non-invasive, examine the entire cell culture, and allow for repeated measurements to be made over time in the same plate.
MEA has also been used for functional phenotypic in vitro screening of patient iPSC-derived neurons.
Read more about electrophysiology:
• High-Throughput Screening of iPSC-derived Motor Neurons on Qube and Qpatch
• Functional Phenotypic Screening of patient iPSC-derived Motor Neurons
Calcium Assays of Neurons
Functional activity of neurons is greatly influenced by prominent calcium signals that regulate neurotransmitter release and membrane excitability. Calcium signaling can be examined in a high-throughput manner with the use of fluorescent calcium-sensitive dyes. The relative fluorescence emitted by these dyes increases when bound to calcium and, once loaded into cells, provides a proxy for changes in cytoplasmic calcium concentrations. Image-based detection systems are available that support a wide range of formats including 96, 384, and 1536-well plates.
Changes in calcium concentration are closely tied to neuronal activity as action potentials are associated with large pre-synaptic calcium influx and a notable rise in postsynaptic calcium at excitatory synapses. This can be observed experimentally by chemical depolarization or electrical stimulation of neurons. Here, electrically evoked calcium responses are observed in the presence of various compounds.
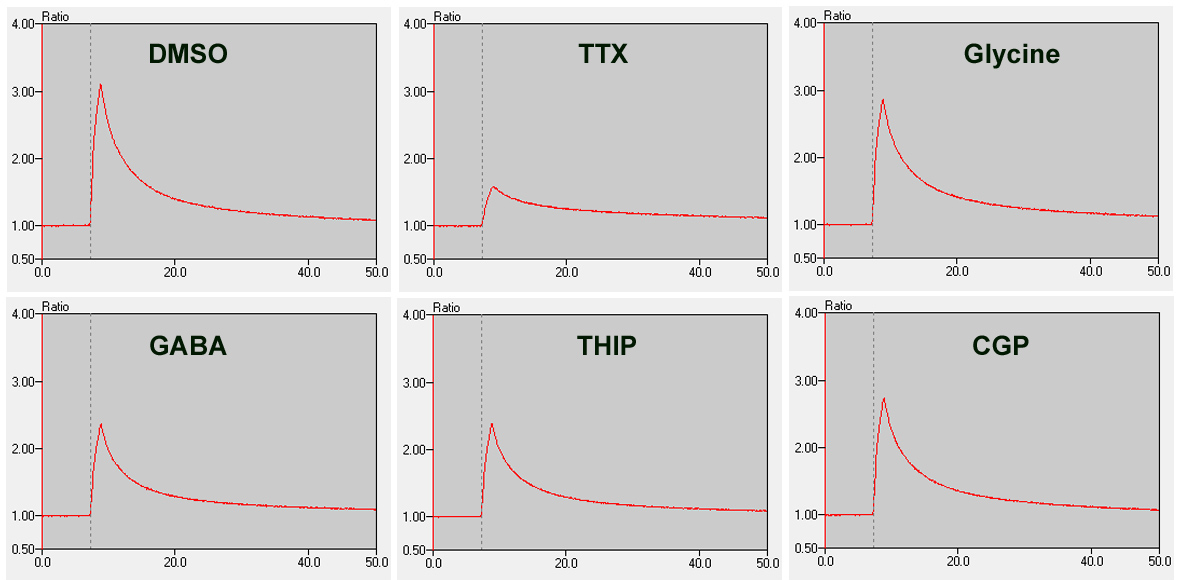
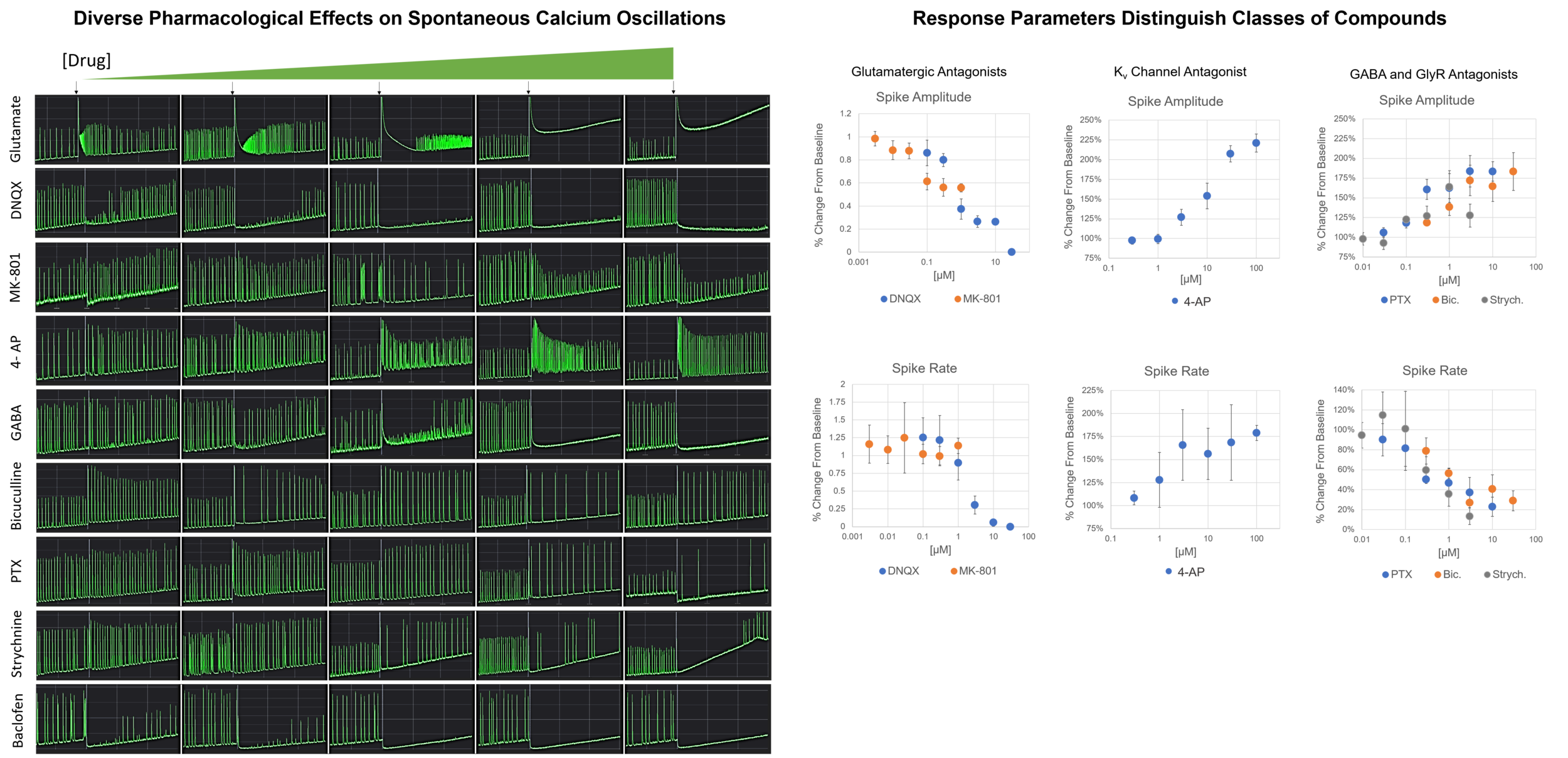
Spontaneous calcium signals can also be observed in vitro when neurons are cultured under suitable conditions to form mature networks that exhibit spontaneous oscillations. Such oscillations are reflective of a population of neurons having synchronous network activity. This system can be used to screen pharmacological agents that alter neuronal activity, as demonstrated below using Brainxell’s Mixed Cortical Neurons (BX-0500).
Read more about calcium assays:
• Pharmacology of Calcium Oscillations in Human Mixed Cortical Neurons
Viability/Cytotoxicity Assays
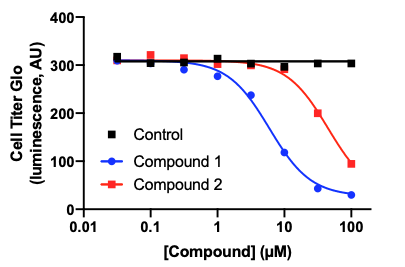
Whether looking at toxicity of a compound, studying a disease phenotype, or exploring cellular mechanisms, assessing the viability of your cell culture can be a critical tool. A variety of cell viability assay kits are commercially available that are based on colorimetric or luminescent reagents, making readouts straightforward. There are also cytotoxicity assays that can assess cell membrane damage, such as an LDH assay, or measure apoptosis, such as a TUNEL assay. In this example, spinal motor neurons (BX-0100) cultured in 96-well plates were treated with 2 different compounds for 1 day and viability measured using a CellTiter-Glo® Luminescent Cell Viability Assay (Promega). This assay provides a luminescent signal that is proportional to the amount of ATP present in the culture. The amount of ATP, an indicator of metabolically active cells, is directly proportional to the number of living cells present in culture.
High-Content Analysis of Neurons
BrainXell’s neuronal products are widely used in high-content analysis (HCA), high-throughput screening, and large-scale automated live-cell imaging. HCA uses multi-parameter image processing and visualization tools to extract quantitative data from cell populations such as spatial distribution of proteins or cell structures.
HCA has been employed for screening of environmental toxins using iPSC-derived motor neurons that were gene-edited to ubiquitously express eGFP (Catalog number BX-0101). Following optimization of culture conditions in a 384-well format and imaging parameters for neuron number and neurite length, high-content imaging yielded a sensitive and robust system with a Z-prime value greater than 0.5.
The detection of neurons and neurites are demonstrated here. Original images (A) were processed to detect cell bodies (B) and neurites (C) for the control (DMSO) and 20 µM rotenone conditions. Dose-response curves for both neuron number (E) and total neurite length per well (F) were measured.
Read more about high-content analysis:
• High-Content Imaging of Motor Neurons for Toxicity Screening
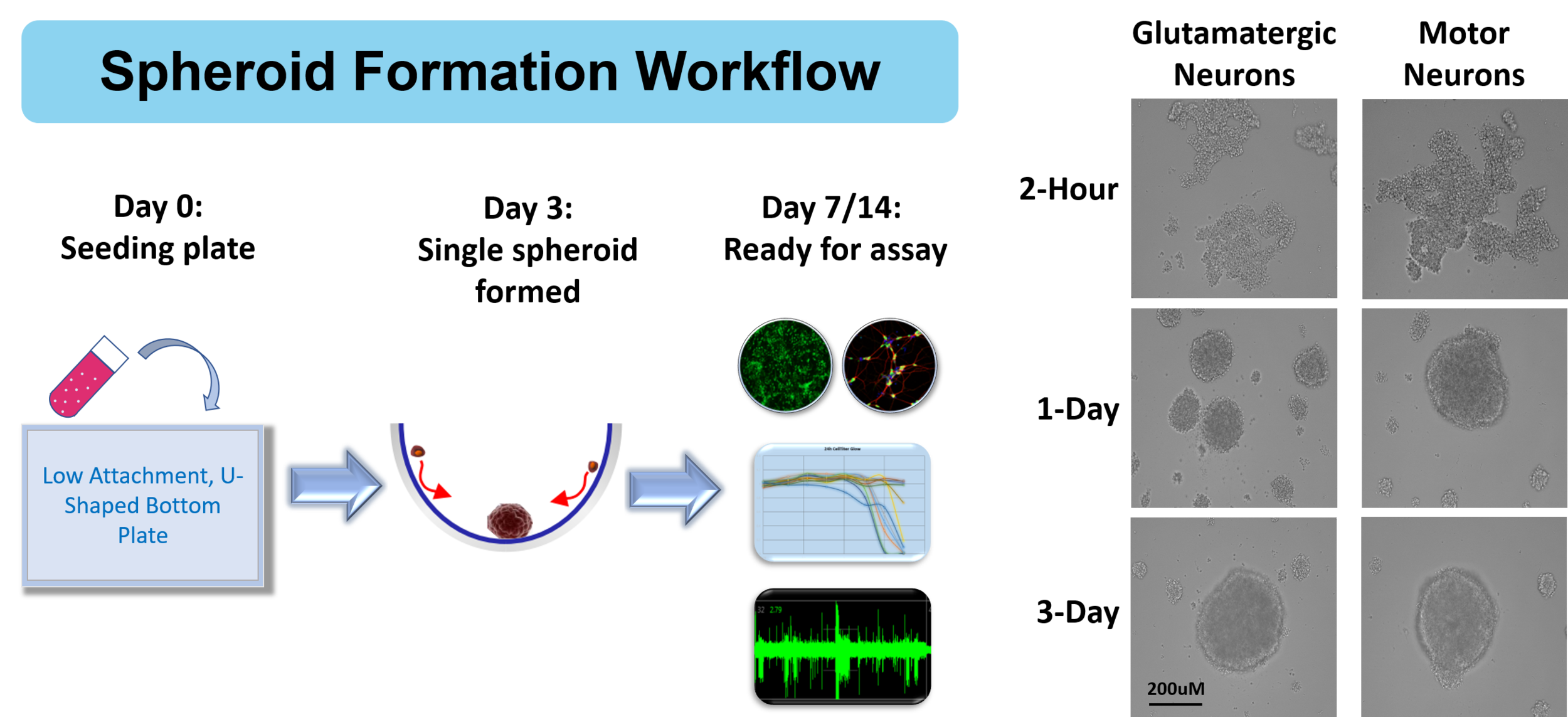
3-D Neural Spheroids
In order to develop the most relevant model systems for studying cell physiology, disease modeling, and drug discovery, many scientists are exploring three dimensional (3D) cultures. Such cultures provide a microenvironment, cell-to-cell interactions, and biological processes that may better represent in vivo conditions.
Read more about 3-D neural spheroids:
• 3D Spheroid Culture Workflow using Human iPSC-derived Neurons